What is oxidation-reduction potential (ORP)?
Category:
Time of issue:2021-11-04
1. The Concept of ORP
ORP stands for oxidation-reduction potential.
It is the difference between the oxidation-reduction potential of the indicator electrode and the reference electrode in a liquid, providing a comprehensive indicator of the oxidation-reduction state of the entire system.
· A low ORP value indicates a high content of reducing substances or organic pollutants and low dissolved oxygen concentration in the wastewater treatment system, with a reducing environment prevailing.
· A high ORP value indicates a low concentration of organic pollutants and a high concentration of dissolved oxygen or oxidizing substances in the wastewater, with an oxidizing environment prevailing.
Traditional oxidation-reduction water treatment technologies have shortcomings such as imprecise control conditions, reagent waste, and environmental unfriendliness. However, using ORP measuring instruments and utilizing the ORP's electrical signal as a detection and control method can significantly improve the precise control level of oxidation-reduction water treatment technology, thereby enhancing treatment efficiency.
Its detection principle is similar to pH; many online pH detectors have dual-channel detection, including an ORP detection channel.
In summary, ORP is an important direction for the development of automatic control technology and precise anaerobic control in wastewater treatment plants, playing a significant role in energy saving, controlling the metabolic pathways of anaerobic microorganisms, and improving treatment efficiency.
2. Difficulties and Influencing Factors of ORP
Due to the numerous oxidation-reduction reactions occurring in wastewater treatment and the varying factors affecting ORP in different reactors, it is difficult to determine which factor is primarily causing the change in ORP.
For example, many organic substances exist in activated sludge treatment systems. Significant changes in organic matter concentration cause minor changes in ORP, but it is difficult to determine which organic matter is primarily causing the ORP change.
Therefore, before studying the indicative role of ORP changes on wastewater treatment, it is necessary to understand the factors influencing these changes.
1. Dissolved Oxygen (DO)
DO represents the amount of oxygen dissolved in water. In aerobic tanks, the DO at the outlet should be controlled at 2 mg/l, and 4 mg/l for pure oxygen aeration. The DO in anoxic denitrification tanks should be 0.5 mg/l. In anaerobic tanks, molecular oxygen is essentially absent, and nitrate nitrogen should be less than 0.2 mg/l.
As an oxidant in wastewater treatment, DO is the most direct cause of increased system ORP. In pure water, ORP has a linear relationship with the logarithm of DO, with ORP increasing as DO increases.
2. pH
pH is an important control factor in wastewater treatment. The optimal growth pH for aerobic microorganisms and acid-producing fermentative bacteria is 6.5-8.5, while the optimal pH for anaerobic methanogens is 6.8-7.2. pH is generally controlled by adding alkali.
The metabolic activities of microorganisms significantly affect pH. During the acid production phase, acid-producing bacteria decompose macromolecular organic matter to produce fatty acids and carbon dioxide, lowering the pH, but the production of ammonia during protein decomposition raises the pH. During the methane production phase, methanogens utilize acetic acid to produce methane, increasing the system's pH.
pH is a significant factor causing ORP to rise or fall. Higher pH values result in lower ORP, and lower pH values result in higher ORP.
It is worth mentioning that although pH and ORP have a certain correlation in wastewater, due to the influence of microbial activity and dissolved oxygen on ORP, the correlation between pH and ORP is not as strong as in pure water.
3. Temperature
Temperature is a crucial indicator in wastewater treatment. Aerobic microorganisms are most active at 15-30℃, while anaerobic microorganisms have optimal temperatures around 35℃ and 55℃.
In anaerobic wastewater treatment, temperature changes significantly affect microbial composition and proliferation, methane production rate, and sludge settling performance. Therefore, to ensure stable anaerobic tank operation, wastewater is generally adjusted to 35℃ or 55℃ before entering the anaerobic tank using cooling towers and steam heating.
Research shows that higher solution temperatures result in lower ORP. This is also true in wastewater treatment. The higher the temperature in the water treatment process, the lower the ORP, which is also related to the smaller water molecule clusters caused by increased temperature.
In addition, temperature changes can simultaneously lead to changes in acidity, gas solubility, biological activity, and the interphase equilibrium of water pollutants, thus affecting ORP.
4. Microbial Composition
Unique ecosystems exist in wastewater biological treatment systems.
In two-phase anaerobic bioreactors, acid-producing bacteria and methanogens are effectively separated, facilitating system control and management. In UASB systems dominated by flocculent sludge, acid-producing bacteria and methanogens are sequentially screened along the flow direction. In anaerobic granular sludge and anaerobic biofilms, the dominant species transition from acid-producing bacteria to methanogens from the outside to the inside.
In anaerobic reaction systems, DO concentration and ORP must be kept very low, especially during the methane production phase, where the oxidation-reduction potential should not exceed -330 mV.
While DO inevitably exists in the influent, due to the unique ecosystem, the synergistic and symbiotic effects of aerobic, facultative, and anaerobic microorganisms quickly reduce the system's ORP to a range suitable for methanogen growth. This low oxidation-reduction potential phenomenon exists not only in anaerobic reactors but also in the flocculent sludge in aeration tanks.
5. Microbial Activity
The activity of anaerobic activated sludge can be represented by the maximum specific methane production rate and the maximum specific COD removal rate. The activity of aerobic activated sludge can also be represented by the maximum specific COD removal rate.
The higher the activity of microorganisms, the faster the rate of oxygen consumption and the rate of production of reducing substances, and the faster the ORP decreases.
ORP, as a comprehensive indicator reflecting the macroscopic redox potential of water bodies, has many influencing factors. In addition to the above main influencing factors, there are also influences from pressure, organic matter, solid substances, and types of microorganisms.
These factors are not isolated; they influence and constrain each other. Therefore, the redox potential of water is also the result of the combined action of multiple factors.
3. Application of ORP in Wastewater Treatment
Earlier, redox potential was mainly used in the treatment of industrial wastewater, especially in the treatment of wastewater produced in metal finishing. Later, it has been widely used in municipal wastewater treatment plants.
Various valence ions and dissolved oxygen, i.e., multiple redox couples, exist in the wastewater system. Through an online ORP monitoring instrument, the redox potential in wastewater can be detected in a short time, eliminating the need for laboratory sampling and measurement. This significantly shortens the laboratory process and improves work efficiency.
Important redox reactions in wastewater treatment systems include the biodegradation of organic pollutants containing carbon, nitrogen, and phosphorus; hydrolysis and acidification of organic matter; nitrification and denitrification reactions; anaerobic phosphorus release; and aerobic phosphorus uptake.
1. Different redox potentials are required by microorganisms at different stages of wastewater treatment.
Generally, aerobic microorganisms can grow above +100 mV, with the optimum being +300 to +400 mV; facultative anaerobic microorganisms perform aerobic respiration above +100 mV and anaerobic respiration below +100 mV; obligate anaerobic bacteria require -200 to -250 mV, with obligate anaerobic methanogens requiring -300 to -400 mV, and the optimum being -330 mV.
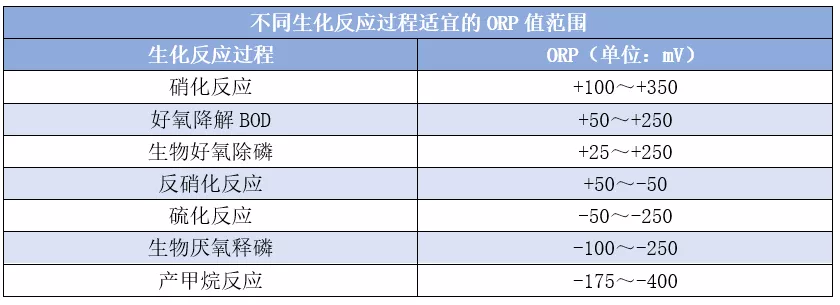
The normal redox environment in an aerobic activated sludge system is between +200 and +600 mV. The suitable ORP ranges for common reaction processes in wastewater biotreatment are shown in the table below:
2. As a control strategy in aerobic, anoxic, and anaerobic biotreatment
By monitoring and managing the ORP of wastewater, operators can artificially control the occurrence of biological reactions. By changing the operating conditions of the process, such as:
· Increasing aeration to increase dissolved oxygen concentration
· Adding oxidizing substances to increase redox potential
· Reducing aeration to reduce dissolved oxygen concentration
· Adding carbon sources and reducing substances to lower the redox potential, thereby promoting or inhibiting the reaction.
Therefore, operators can use ORP as a control parameter in aerobic, anoxic, and anaerobic biotreatment to achieve better treatment effects.
Aerobic biotreatment:
ORP has a good correlation with COD removal and nitrification. Controlling the aerobic aeration amount through ORP can avoid insufficient or excessive aeration time, ensuring the quality of the treated effluent.
Anoxic biotreatment:
ORP and the nitrogen concentration in the denitrification state have a certain correlation during anoxic biotreatment, which can be used as a criterion to judge whether the denitrification process is complete. Relevant practices show that during denitrification, when the derivative of ORP with respect to time is < -5, the reaction is more thorough. The presence of nitrate nitrogen in the effluent can prevent the generation of various toxic and harmful substances, such as hydrogen sulfide.
Anaerobic biotreatment:
During anaerobic reactions, when reducing substances are produced, the ORP value decreases; conversely, when reducing substances decrease, the ORP value increases and tends to stabilize within a certain period.
In summary, for aerobic biotreatment in wastewater treatment plants, ORP has a good correlation with the biodegradation of COD and BOD, and with nitrification reactions.
For anoxic biotreatment, ORP and the nitrate nitrogen concentration in the denitrification state have a certain correlation during anoxic biotreatment, which can be used as a criterion to judge whether the denitrification process is complete.
3. Control the treatment effect of the phosphorus removal process and improve the phosphorus removal effect
Biological phosphorus removal includes two steps:
· First, in an anaerobic environment, during the phosphorus release stage, fermentative bacteria produce fatty acids under the condition of ORP at -100 to -225 mV. The fatty acids are absorbed by polyphosphate-accumulating organisms (PAOs), and phosphorus is released into the water body.
· Second, in the aerobic tank, PAOs begin to degrade the fatty acids absorbed in the previous stage and obtain energy from the conversion of ATP to ADP. This energy storage requires the adsorption of excess phosphorus from the water. The phosphorus adsorption reaction requires an ORP of +25 to +250 mV in the aerobic tank for biological phosphorus storage to occur.
Therefore, operators can use ORP to control the treatment effect of the phosphorus removal process and improve the phosphorus removal effect.
When operators do not want denitrification or nitrite accumulation to occur during a nitrification process, an ORP value exceeding +50 mV must be maintained. Similarly, to prevent odor (H2S) generation in the sewer system, operators must maintain an ORP value exceeding -50 mV in the pipeline to prevent the formation and reaction of sulfides.
4. Adjust aeration time and intensity to save energy and reduce consumption
In addition, operators can also use the significant correlation between ORP and dissolved oxygen in water to adjust the aeration time and intensity of the process, achieving energy saving and consumption reduction while meeting the conditions of biological reactions.
In summary, ORP detection methods are simple, equipment prices are low, measurement accuracy is high, and detection data is displayed in real time.
Through online ORP detection, operators can quickly grasp the wastewater purification reaction process and water pollution status information based on real-time feedback, thereby achieving refined management of wastewater treatment and efficient management of water environmental quality.
However, as mentioned above, numerous redox reactions occur in wastewater treatment, and the factors affecting ORP vary among different reactors.
Therefore, in wastewater treatment, operators also need to further study the correlation between dissolved oxygen, pH, temperature, salinity, etc., and ORP in the water based on the actual situation of the wastewater treatment plant, and establish ORP control parameters suitable for different water bodies.
Tags:
-- Recommended --
Shijiazhuang Tianwang Environmental Protection Technology Co., Ltd.
Shijiazhuang Tianwang Environmental Protection Technology Co., Ltd. is a high-tech enterprise specializing in the research and development, manufacturing and sales of water treatment equipment.
Contact Information
Production address: No. 9, Fengchan Road, Economic and Technological Development Zone, Shijiazhuang City
Office Address: 25th Floor, Block C, No. 310 Changjiang Avenue, Shijiazhuang High-tech Development Zone
Contact Number:
0311-89272359 0311-68039237
Enterprise Email:
twhbkj@163.com
Website: en.sjztwhb.com







